UNIFIED WORKSPACE
One environment for SAS, R, and modern languages
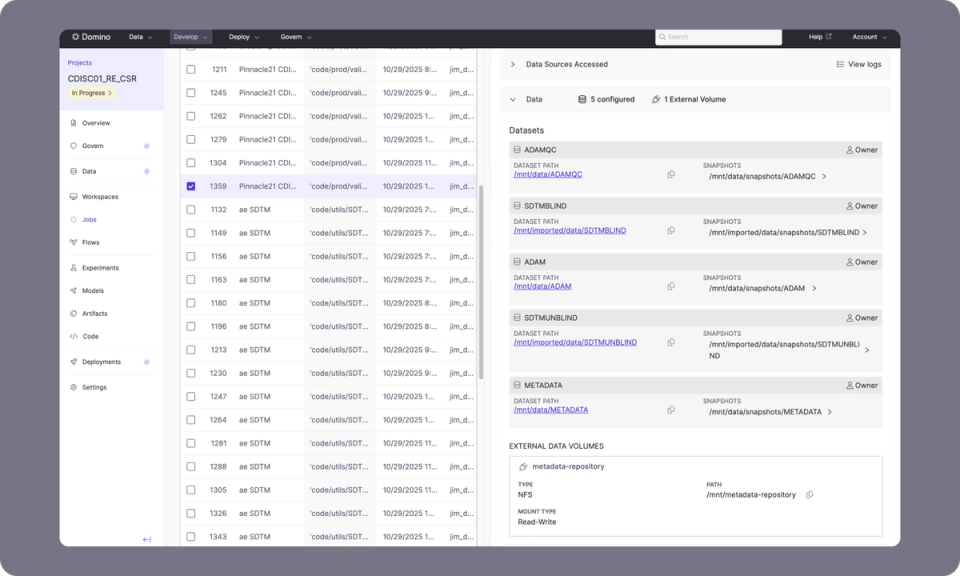
Domino SCE brings languages together in one space for analysis, review, and submission. Programmers work in their preferred IDEs, eliminating retraining and disruption caused by forced tool changes. Shared templates and standards keep outputs consistent across studies.